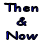1900 LZ-1 filled with Hydrogen
Photo credit: Public domain, from air-ship.info.
This famous photo shows the LZ-1 on her first flight, 2 July, 1900. Little is said about this photo, but there is much to be said! Indeed, there is a whole page on this site dedicated to the LZ-1 here: Luftschiffbau Zeppelin 1, (LZ-1).
It's photos like this and of ALL the other airships, famous and lesser known, from the early 1900's that always made me wonder "Where did they get the hydrogen?" I know, for example, that Alberto Santos-Dumont strongly desired his own "hydrogen generation plant", ("My Airships", Santos-Dumont, 1904, pgs 129 & 137), and that all the experimenters of the day needed quite a large volume of hydrogen for each of their airships. I assumed each of these "independent" airship builders could not depend on a nation to set up a hydrogen source for them the way the Nation-States (Germany, France, England, United States) could do as they developed their truly giant airships - out of the reach of the "back-yard" developer! Indeed, where did Willows, 1905, at age 19 get his hydrogen at Cardiff UK? Or American Thomas S. Baldwin? (Roy Knabenshue, the 1st American to fly a powered airship, piloted an airship built by Thomas S. Baldwin. Knabenshue then went on to build and fly his own.)
It took a bit of sleuthing! After all, even today, well over 100 years from the age of the airship, hydrogen is not an easy-to-find commodity! Try to find anyone today who has any clue how to produce large volumes of hydrogen in one's own garage! The greatest source (tongue-in-cheek), "YouTube", is only going to reveal methods producing minuscule amounts of hydrogen such as the well-known electrolysis of water using a battery. You're not going to fill a 7,000 cubic foot airship using YouTube techniques! (Well, you COULD use electrolysis but be prepared for an ENORMOUS electric bill!)
So how did they do it?
How did they get so much hydrogen?
A couple of clues in my research led me to the answer. First, there was a citation in the August 28, 1909 "Flight" magazine that said:
Spherical balloons, such as are used for the purpose of pleasure trips, are, on the score of cheapness, inflated with coal gas from the ordinary town supply, the lifting power of this medium is so moderate, only about 30 lbs. per 1,000 cubic feet, that it is to all intents and purposes useless for dirigibles, which have to carry much greater loads in proportion to their size.
"Coal gas" or "Town Gas" was the common gas delivered to homes for illumination and heating in the late 1800's. Urbanization had led to the production of coal gas as a lighting and heating source and its production and use persisted through the mid 1900's until it was replaced by Natural Gas. Coal gas is a mixture of gases (chiefly hydrogen 50%, methane 35%, and carbon monoxide 10%) obtained by the destructive distillation of coal. Density of coal gas is a little less than half that of air, so it worked fairly well in spherical balloons which did not have much structural weight.
Coal gas with its large percentage of hydrogen remains somewhat lighter than air, while natural gas in our time (a composition of gases, mainly methane) delivered to homes today contains only a trace or no hydrogen and the composition's density is about 0.7, just under 2/3 the density or air so it does remain "lighter than air", but would not be very effective in balloons or airships. ("Propane" used in home outdoor appliances, campers and rural homes is sometimes confused with "natural gas" but propane is much denser than air and thus "heavier than air".)
The point is, as the 1909 "Flight" magazine article made clear in a single short paragraph is that while "coal gas" was readily available in the era of early airships, it was not suitable for airships! A 7000 cubic foot airship could only lift about 200 pounds, total, and excluding the weight of the pilot, that means the entire vehicle; gas bag, rigging, keel, engine, propeller, ballast, etc. could not weight more than 30 pounds or so! There is no way the airships built in the early 1900's were going to weigh less than 30 pounds such that coal gas would be used as the source of their buoyancy! They needed hydrogen!
The "big boys", with money that is, Nation States, could afford the "The Messerschmidt Contact Process". In that process, steam is passed over red-hot iron which is decomposed into its elementary constituents, hydrogen and oxygen. The oxygen is taken up by the iron forming iron oxide, while the hydrogen is liberated. The resulting iron oxide is reconverted into iron by treating it with reducing gases, which produces carbon dioxide and water (steam). The process is therefore reversible. (This is from Aerial Age Weekly, 6 November, 1916, pg 199.)
Other clues came from a couple of other sources. Here is a citation from Walter Wellman’s 1911 book “The Aerial Age”, page 131:
"A staff of engineers and experts was organized, including M. Gaston Hervieu, gas engineer, Alexander Liwenthaal, an architect, and M. Colardeau, a mechanical expert. In Norway I chartered a steamship to carry us to Spitzbergen, again securing the old Frithjof, which had taken us to Franz Josef Land in 1898. A hydrogen gas apparatus of large capacity was built in Paris to be transported to Spitzbergen. One hundred and ten tons of sulphuric acid for making hydrogen were ordered from Reher and Ramsden, Hamburg, and seventy tons of iron turnings were secured in Norway. Tons of provisions were purchased from Armour & Co., Chicago, and Acker, Merrall & Condit, New York, and shipped across the Atlantic."
Another clue is found in this photo of the almost completely unknown Trombly-Haddock airship, the "Bullet":
Photo credits: chezpeps.free.fr collection
If you can't quite read it, the sentence in the lower-right corner says: "To fill the envelope required five tons of sulphuric acid and four tons of iron filings, costing 175 dollars." That pretty much cinched it - I'm looking for "sulphuric acid and iron filings" as the method of hydrogen production as the method the early aviators used!
Knowing I was looking for sulphuric acid and iron filings as the source of hydrogen I set out looking for the process. I quickly found a very nice article in a 1908 edition of "Popular Mechanics", and rather than try to summarize it here, I'm presenting the article, in its entirety as published.
(In the article below, “degree Bé” refers to the Baumé scale, a measure of a solution's specific gravity. The Baumé scale is almost never mentioned in chemistry course but tradesmen use it as a convenient way to check solution concentration.)
Here's the article, from the June, 1908 "Popular Mechanics", Part II of “Building Airships and Flying-Machines”, by Carl Shelley Miner, The Miner Laboratories, page 381. (Part I was in the May, 1908 and is interesting in its own right as a description of "Building Airships and Flying-Machines, How to build a dirigible balloon", by Glen Curtis!)
BE sure to read past the following article Popular Mechanics for a wonderful addition about the Hydrogen Plant of Russian airship experimenter Konstantine Danilewsky, from 1897 to 1900! His process paid for itself!
Popular Mechanics, June 1908, pages 381-383
Building Airships and Flying-Machines, Part II, How to make a hydrogen gas generator
Notwithstanding recent promising developments of the aeroplane, especially the work of Farman, it is still true that the bulk of the really satisfactory results in aerial navigation have been secured by the use of the power-driven gas bag. While in many directions there have been great improvements on the balloons of former times, hydrogen gas generated by means of iron and sulphuric acid is still used to fill them, as it has been almost from the beginning of ballooning. A few attempts to use hot air have been made, but it is so inferior to the hydrogen that its use has never become general. The hydrogen gas, because of its extremely low gravity, the ease with which it can be produced and its cheapness, stands without a rival. It is so absolutely without competition in this field that there seems not the slightest probability of its ever being displaced. The efforts of those who are working on the problems of aerial navigation have been directed therefore not to discovering a substitute for it, but to evolving cheaper and more convenient methods for its production.The method commonly employed consists in treating iron shavings with sulphuric acid. The increasing use of hydrogen in airships and, even more, its use in certain industrial processes, such as welding, have drawn the attention of various investigators to the problem of improving this process, and several excellent methods, some of them new, have recently come into use. In the Russian-Japanese war the Russians produced gas for their balloons by treating aluminum with an alkali. This is a more expensive method than the use of iron and sulphuric acid, but has the advantage that the aluminum is lighter than the iron and that the alkali may be obtained in solid form, and so can be transported and handled much more easily than sulphuric acid. Recently metallic silicon has been substituted for aluminum in this process.
The French transport the hydrogen for their war balloons compressed under a pressure of 185 atmospheres in metal cylinders and have found this plan very satisfactory.
Two new methods make use of solids which, when brought in contact with water, generate hydrogen gas. One of these manufactured in this country, is an alloy of lead and sodium. The reaction of metallic sodium when brought into contact with water is well known to even the most superficial student of chemistry. Hydrogen is evolved and caustic soda is formed. The object of using the lead sodium alloy is to obtain a material which is less violently reactive than the sodium alone, for even a small amount of moisture may cause it to react with explosive violence. The lead sodium alloy may be more easily and safely handled and is a convenient means of generating hydrogen.
Another material which is used in somewhat the same way is calcium hydride. This compound is formed by passing hydrogen over heated metallic calcium and is a solid similar in nature to calcium carbide. By treating it with water, hydrogen may be generated just as acetylene is generated from calcium carbide. One pound of calcium hydride is capable of producing 17 cu. ft. of hydrogen gas, and when it is realized that 1lb. of iron and 2 lb. of ordinary commercial sulphuric acid produce only 6 1/3- cu. ft. of gas, the immense superiority of calcium hydride will be apparent at once. The product is, however, so high priced that it is not practical for the ordinary investigator of the problems of aerial navigation. The old iron and sulphuric acid method is still the best available, and it behooves him to understand it thoroughly.
Since hydrogen is the lightest of all gases, it follows that the purer the hydrogen, the more suitable it is for filling balloons. Air is 13 times as heavy as hydrogen; carbon dioxide, 22 times as heavy; hydrogen sulphide, 16 times; sulphur dioxide, 32 times. All these gases may occur in the hydrogen produced by the use of iron and Sulphur in acid. When a very pure hydrogen is required, as in the case of Andre's balloon or Wellman's airship, great precautions are taken to remove all these impurities as well as the water and sulphuric acid which may be carried along with the gas mechanically. The accompanying sketch shows an apparatus which was designed for Andre:
C, (Fig. 1) is a lead lined mixing tank, where the 60 degree Bé acid is added to sufficient water to reduce the gravity to 16 degree Bé. The acid is then run into the generator, B, into which the iron is introduced through the valve X. A stop cock should be attached to the base of the generator, B, for the purpose of removing the sludge. The gas passes from the generator to the washing chamber, D, where it is thoroughly washed by fine sprays of water. It then passes through the chamber, E, which contains trays of chemicals for removing the residual impurities. Finally it goes to the testing chamber, F, where its actual purity is tested before it is passed on into the balloon.
The apparatus used by Wellman is similar. The gas is thoroughly washed and is passed through a cylinder filled with caustic soda, which removes acid and carbon dioxide. It is afterwards dried, tested and perfumed, the latter being an important precaution to aid in the prompt discovery of leaks, since hydrogen itself is odorless.
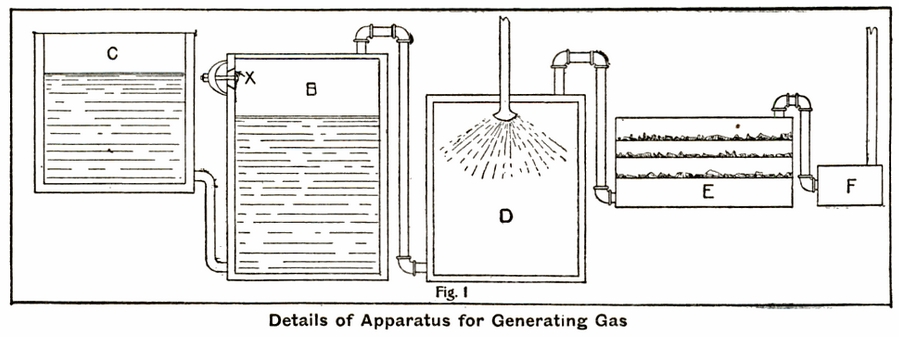
For filling ordinary balloons or airships such precautions are unnecessary. A simple apparatus such as is shown in the accompanying sketch (Fig. 2) will produce a gas quite satisfactory for the purpose. 'A' is an ordinary 50 gal. barrel, having a pipe, B, connecting it with the lime tank, C. 'A' should have an opening in the head through which the iron and acid may be introduced and which admits of being tightly closed by a bung or valve. It also has an outlet, O, near the bottom, for the removal of sludge and exhausted acid liquor. 'C' is another barrel partially filled with milk of lime. This will remove acid and carbon dioxide. The removal of the acid from the gas is very important, as otherwise it may injure the envelope of the balloon. 'C' has a flexible connection, H, which may be ordinary hose, through which the purified gas passes to the bag. The iron should be scraps or shavings, not large chunks nor fine powder. The ordinary 60 degree commercial sulphuric acid may be used, but should be diluted to 16 degree. This may be done by adding it to about 3 1/3 times its own weight of water. Add the acid to the water slowly, with constant stirring. Do not add the water to the acid, as that procedure has a tendency to reduce the surplus population *.
For a bag of a capacity of 5,000 cu. ft., approximately 900 lb. of iron and 1,800 lb. of 60 percent acid will be required. The actual operation of generating the gas is quite simple. It consists merely in filling the generator barrel about one-third full of iron, adding dilute acid to within about 1ft. of the top and immediately closing the opening tightly; the gas then passes through the pipe to C, and after being purified by passing through the lime water is introduced into the balloon through the flexible tube H. Several generators should be ready, and as fast as one is exhausted it should be disconnected and a new one connected and put into operation. The exhausted generator should now be emptied of the acid liquor and sludge through the outlet O, and then refilled with acid, this procedure being repeated until all of the iron has been used up. Of course, as many generators may be used simultaneously as the operator desires in order to hasten the filling of the bag.
There are many more expensive forms of apparatus for this purpose, but the one here described will be found quite effective and very cheap. The barrels should be of hardwood and tight. All the connections should be close and the bung which closes the inlet and the generator should be accurately fitted.
Remember that hydrogen gas mixed with air is violently explosive and do not allow a light or a fire of any sort near the generator or gas pipe.
-----------------------
*Ed: For those unfamiliar with this meaning, adding acid to water is safer than adding water to acid. Adding water to acid tends to result in an explosion, with deadly results. To help remember, there is an old mnemonic taught in chemistry class which goes like this: "Do what you oughta, poor acid into wattah." And another one is: "Poor ole Joe, lying calm and placid, for he poured water into acid."
In late 2017 I learned about the experiments of Dr. Konstantine Danilewsky who lived in what is present-day Kharkov, Ukraine and who designed his own very successful hydrogen generator for his airships. He even wrote a whole chapter about his process in his 1900 Russian book "A Steerable Flying Apparatus". Uniquely, the hydrogen Danilewsky produced for his work cost him nothing because he found a way to make the generator pay for itself!
Danilewsky wrote, in 1900, that he was "always amazed that the production of hydrogen is considered an expensive production. Even the Aero-club in Paris has awarded a prize to someone who will reduce the cost of hydrogen. In the past (1898), in my report, I pointed out that the production of hydrogen is almost free of charge; ... At first glance, this seems to be a paradox..." Then he writes "The cheapest method of obtaining hydrogen is an old method: the action of liquefied sulfuric acid on iron filings. The main products of this interaction are pure hydrogen (H2) and iron sulfate (SO4_Fe2)."
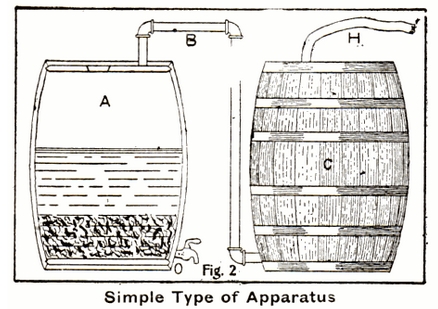
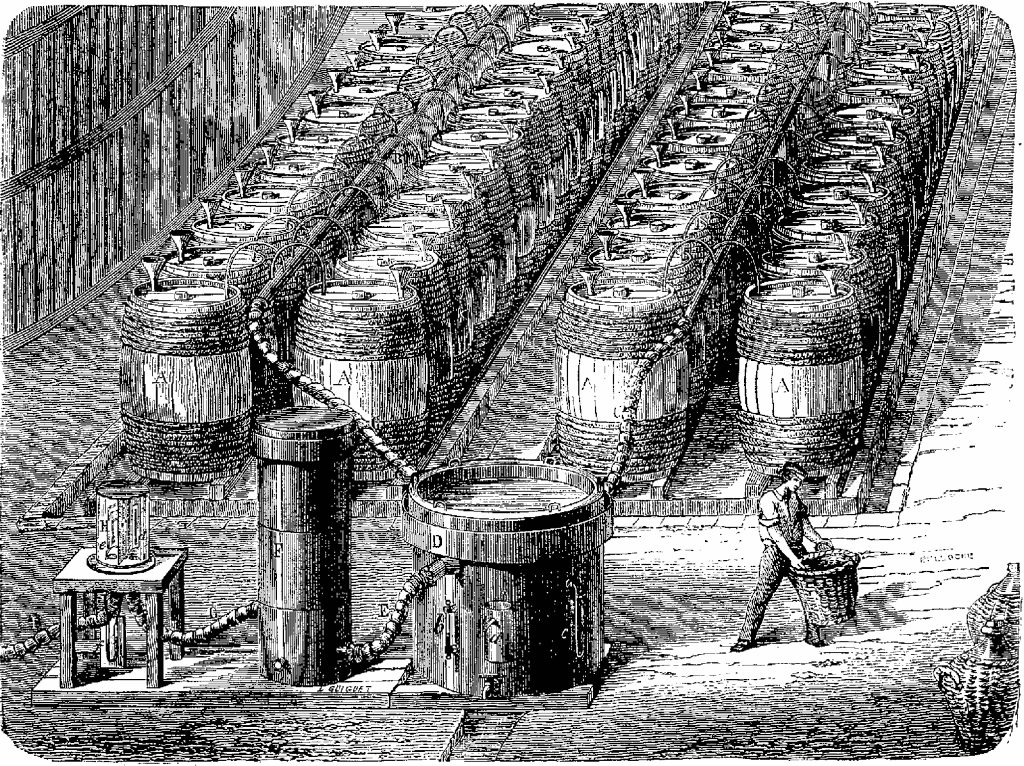
That statement "old method" caught my eye for it took me quite a bit of research to determine the method, and still I found citations only from the early 1900's, and here is Danilewsky saying "...it's an old method"! An email from my friend, Dr. Akimov in the Ukraine, set me straight about my ignorance! It turns out the earliest hydrogen production by this method was in 1650 by Turquet de Mayerne, a Swiss-born physician! There are many engravings of balloon activity in the 1600s to 1800s showing the many barrels and pipes which were set up to produce hydrogen. Here is one such image:
Photo credits: Public Domain, originally from "Fig Tree - The Wonders of Science, 1867 - 1891, Volume 2"
In Danilewsky's book he reveals the secret about his process: He writes "Chemical plants, where iron sulphate is produced in large quantities, look at hydrogen as a waste product while aeronauts, needing the hydrogen, look at the iron sulphate as waste...simple logic will tell you that in the first case, each of them must be given what is valuable to him: the aeronaut to be provided hydrogen, and the chemical plant - iron vitriol. The chemical plant, therefore, will pay the aeronauts all the cost spent on obtaining hydrogen."
("Iron vitriol", also known as "green vitriol" is the iron salt produced from the chemical reaction of sulfuric acid and iron. The product is used as a pigment, as a fertilizer, and medically as an iron supplement.)
In Danilewsky's hydrogen plant, he produced hydrogen for his airships, and green vitrol to sell, thus providing his hydrogen for "free"!
So how did he do it? Chapter 5 of his book continues:
The calculation is very simple:
In theory, 33 [imperial] pounds [about 16.38 kg] of sulfuric acid, worth 85 kopecks and 23 [imperial] pounds of iron filings, cost 30 kopecks (total 1 rub. 15 k.), provides 89 [imperial] pounds of ferrous sulfate [green vitiol], which sells [ 2.12 kopeck per imperial pound]. ...So, with a cost of 1 rub. 15 kopecks for the raw material, it produces 1 rub. 90 kop. gross income from the sale of the vitriol." (Danilewky obtained his hydrogen for free!)
Danliewsky adapted his hydrogen plant from the plans and drawings of VP Pashkov of the Kharkov Technological Institute.
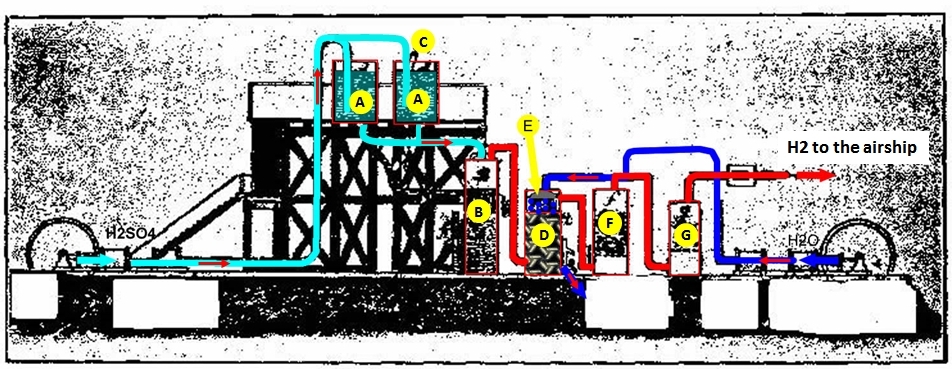
Referring to the figure below (adapted from the insightful interpretation of Dr. Alexander Akimov, of Kharkiv, Ukraine), the hydrogen production is as follows:
Sulfuric acid, properly diluted, is pumped into tanks "A". The acid is regulated by valve "C" (one valve for each tank A), and flows into tank "B" as a spray or "rain" onto the iron fillings in tank "B". This is represented by the light-blue lines in the figure. The hydrogen produced (red line) flows out of tank "B" into the bottom of tank "D". Tank D is filled with coke, through which the hydrogen percolates. When the hydrogen emerges from the coke, it encounters a spray of water, "E" which has been pumped in to the tank (dark-blue lines). The hydrogen, thus "washed" to give up its molecular water, is allowed to pass to tanks "F" and "G" which are filled with caustic lime. This bubbling of the hydrogen through the lime further purifies it (I am no chemist), and the purified hydrogen (red line) is then passed to the airship envelope.
I am not addressing the process to retrieve the iron sulfate and dry it and produce the green vitriol. Suffice it to say that the iron sulfate from tank "B" is drained and processed into the product marketable as the green vitriol.
So here, a decade before the 1908 Popular Mechanics article appeared, Russian aviation enthusiast Konstantine Danilewsky perfected the production of hydrogen for his experiments and made it profitable! It was so simple, wrote Danilewsky, that two "ordinary" townsfolk were hired to operate the hydrogen production with complete success. I should note that in 1897, when Danilewsky began his experimental efforts, he apparently filled his "Embrio" airship with hydrogen produced by a process very much like what is described in the Popular Mechanics article. Danlewsky describes his initial hydrogen generator as "collapsing" just as he obtained enough hydrogen to fill his airship!
There you have it! While some of the airship experimenters in the early 1900's may have been fortunate enough to be able to buy their hydrogen from a producer in the area (such as existed at the France Aéro-Parc (Aéro-Club Français), at Saint Cloud), many more had to use the established technique to produce their own! All they needed were lots and lots of sulphuric acid and lots and lots of iron filings, and a lot of careful, experimental care and patience!